Galleri is a blood test that looks for cancer-specific DNA methylation patterns and suggests a likely tissue of origin for 50 different types of cancer. It is offered as a complement to standard screening and is currently commercially available in the United States (list price: $949).
The idea of diagnosing cancer with a blood test has been pursued for years. If that becomes possible, it will lift the heavy burden of multiple invasive procedures, as cancer screening usually requires one type of procedure for one type of cancer – colonoscopy for colon, cervical swab for cervical cancer. Even the rare less invasive screenings – for example, blood screening (prostate-specific antigen) for prostate cancer, still only reveal one type of cancer. What is worse, for the majority of cancer types, there are no screening methods for early detection at all.
Galleri – a blood-based multi-cancer early detection test (MCED) – promises to detect signals of ~50 cancers from one draw. Today, it is offered as an additional screening test, not a substitute for any other type of procedure.
In reality, multiple manufacturers and modalities are pursuing MCED. But the Galleri test currently leads by scale, with ~140,000 participants in the randomized trial in the UK, and 35,878 enrolled in the US PATHFINDER2 study.
At the ESMO Congress, interim findings from PATHFINDER2 provided new important data.
As of October 2025, Galleri is commercially available in the US (list price: $949). In Canada, some private clinics facilitate access by sending samples to the US lab. In the UK it is available through the clinical trial.
For the new trial, 35,878 participants were enrolled from 50 locations in the US and Canada. In the intermediate analysis, 23,161 were analyzable because those people completed a 12-month follow up period after the test.
What the interim PATHFINDER2 numbers mean in practice
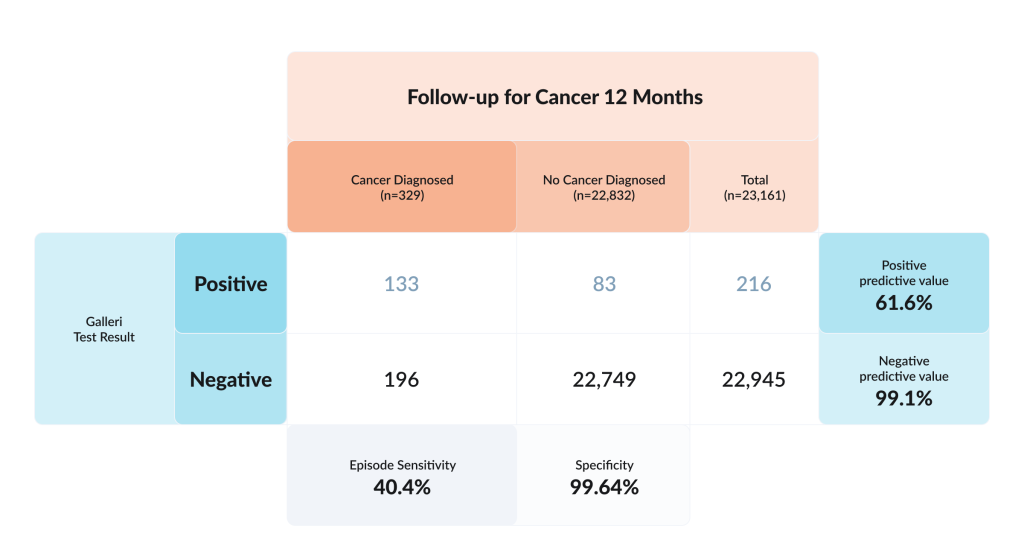
Performance Metrics of Galleri test in people aged 50 and over without suspicious symptoms.
Adapted from: Eric Topol. The Largest Study of a Multi-Cancer Early Detection Blood Test. Ground Truths.
Cancer was confirmed from the Galleri test in 133 cases, or 5.7 cases per 1,000 participants. Early cancer (Stage 1 or 2) was detected in 61 participants, equivalent to 3 cases per 1,000 participants.
AI of the methylation marker pattern correctly identified the site of origin in nearly all (92.7%) participants who had cancer.
There were 196 false negatives – the MCED was negative but the participant was found to have cancer during the 12-month follow-up.
Does a positive test mean the presence of cancer?
The test is not perfect – if the result was positive, there was a 61.6% chance that cancer would be confirmed within a year.
The test was performed in people without specific indications – no symptoms or family history were considered, only the age of 50+ years. In this setting, the cancer detection rate was 5.7 per 1,000 – likely too low to recommend the test for the general population.
What is remarkable, the accuracy in localizing the site of the tumor is 92.7%, which makes diagnostic search much more effective.
If the test is negative
If the test is negative, there is a 99.1% chance that the person does not have cancer. But this number might be misleading – such a high percentage is partly due to the low base rate of cancer in this population. Over a year, ~1.4% of participants were diagnosed with malignancy. With such prevalence, even a moderately sensitive but highly specific test produces many true negatives.
In large numbers, like in the recent study, where data of 23,161 participants were analysed, 196 cancers occurred after a negative result (false negatives), meaning the test missed more cancers than it found. Its sensitivity was about 40%.
Are there other use cases for the test?
Post-treatment surveillance/recurrence monitoring. Biologically attractive, but not what Galleri is validated for today; recurrence use typically relies on patient-specific tumour-informed assays. Still, researchers and payers will watch whether MCED-style methylation adds value in survivors at scale. A review from Mitsuho Imai and colleagues calls for prospective trials before routine surveillance use.
Should indications narrow to higher-risk cohorts?
When screening average-risk population, incidence is low by definition, so absolute detection yields look small even when a test performs well. That argues for targeted indications where incidence of the disease is higher, so we need to perform fewer tests.
A paper from Mussab Fagery and colleagues highlights where MCED adds more value at lower costs:
– Adjunct for under-screened groups. Offer MCED alongside existing programs to people who routinely skip standard tests – e.g. those who are unwilling to undergo colonoscopy; The aim is to capture otherwise missed cancers and then route people who test positive into the usual diagnostics;
– Defined high-risk cohorts. Examples include strong family history and genetic predisposition, relevant occupational/environmental exposure, or risk scores above a threshold. Higher prevalence improves both clinical and economic efficiency;
– Triage after equivocal results. When a standard screen is indeterminate, an additional test can help decide between immediate work-up and short-interval follow-up.
Among MCED candidates, Galleri has translated a methylation-based assay into a usable clinical service fastest; this became possible due to pairing a large prospective evidence program (PATHFINDER → PATHFINDER2; the 140,000-participant NHS-Galleri trial) with operational readiness: ordering, logistics, and interpretive reports. This combination has driven earlier clinical uptake than their peers, even as definitive data are still pending.


